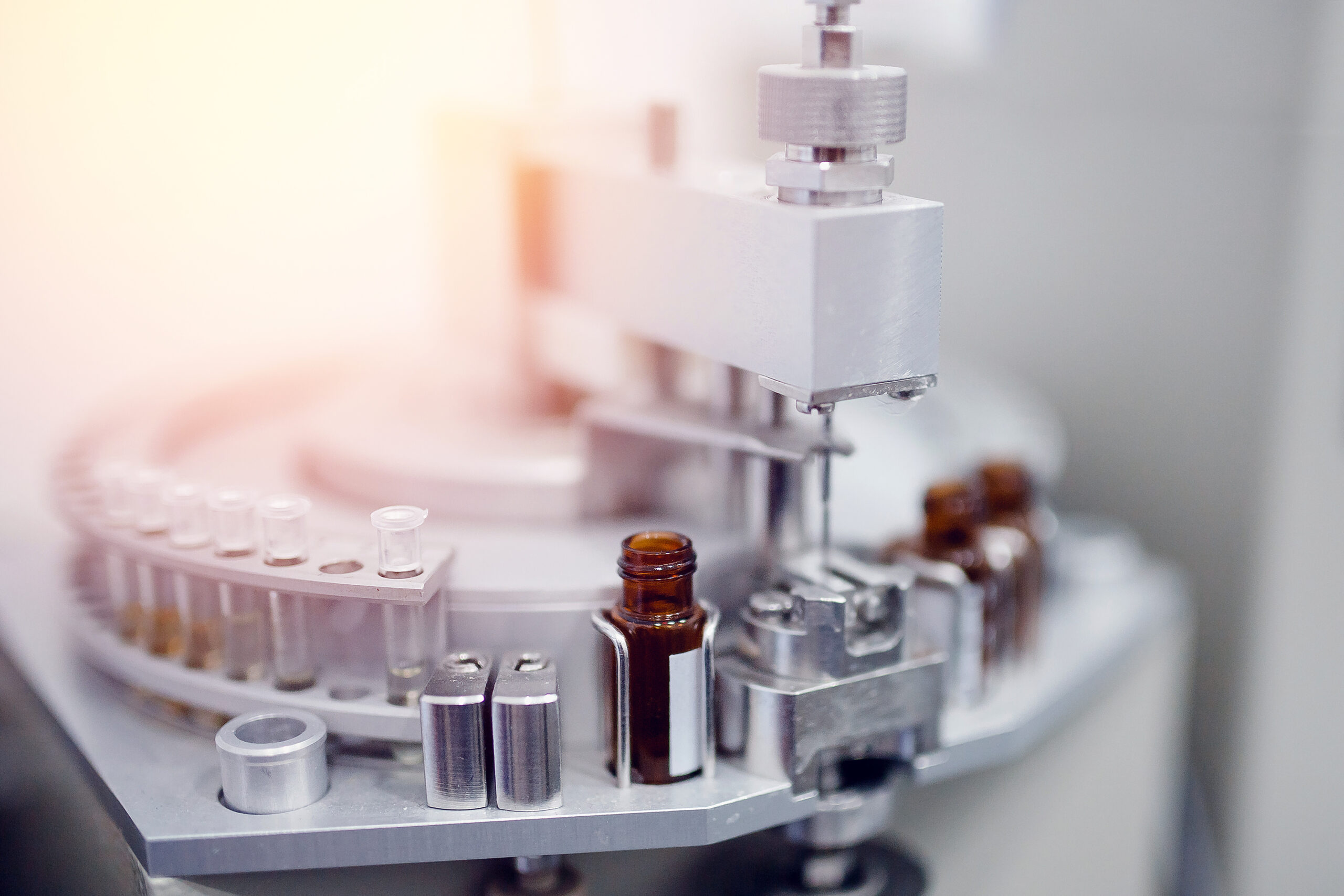
Clinical Trial Pharma Equipment: Driving Agility, Compliance and Innovation
Topics:
September 2025
Introduction
Clinical trials sit at the heart of pharmaceutical innovation. They are the essential bridge between laboratory discoveries and treatments that can transform patient lives. Yet they are also among the most complex and highly regulated phases of drug development. Every process must meet stringent quality standards, timelines are under constant pressure, and the cost of delays can run into millions.
Within this landscape, the right equipment is not a supporting player; it is a critical enabler of progress. At SP Automation & Robotics, we design and deliver bespoke pharmaceutical automation solutions that address the unique demands of clinical trials. By combining agility, reliability and compliance, our systems help pharmaceutical organisations bring therapies to patients more efficiently and with greater confidence.
The Unique Demands of Clinical Trial Manufacturing
Manufacturing within a clinical trial setting is unlike any other stage of pharmaceutical production. Traditional large-scale production systems are often unsuited to the variability and agility required. Instead, equipment must be specifically designed to address challenges such as:
Small Batch Sizes
Early-phase trials may require only a few hundred or even dozens of units. Running these on full-scale manufacturing systems leads to inefficiency, wasted materials and unnecessary costs. Clinical trial equipment must be capable of delivering consistent results in small quantities without compromising quality.
Frequent Changeovers
Unlike commercial production, clinical trial protocols can shift quickly. A system may need to move from one formulation or packaging format to another in a matter of hours. Equipment designed for agility, with rapid changeover features, can drastically reduce downtime and keep trial timelines on track.
Space Limitations
Research and development facilities rarely have the luxury of expansive production floors. Equipment must be designed to fit seamlessly into constrained environments while still delivering full functionality. Compact footprints and modular designs are increasingly vital.
Regulatory Compliance
Clinical trial data must meet the highest levels of scrutiny. Every unit produced must be traceable, and every process must be validated. Equipment used in trials must therefore include features such as digital data capture, secure audit trails and the ability to meet international regulatory frameworks, including MHRA, EMA and FDA requirements.
Cost Control
With escalating R&D costs across the pharmaceutical industry, efficiency at the trial stage is paramount. Bespoke equipment that reduces waste, minimises downtime and optimises workflows can deliver substantial long-term savings.
Why Bespoke Clinical Trial Equipment is the Future
A one-size-fits-all approach no longer serves the pharmaceutical industry. Each trial has its own specific requirements, and these demands evolve as the therapy progresses from Phase I through to Phase III and eventually into commercialisation.
Bespoke automation solutions offer several decisive advantages:
Custom Fit for Each Environment – whether the challenge is space, workflow or a unique trial protocol, bespoke equipment can be designed to meet the exact needs of the trial facility.
Scalability – modular equipment allows systems to expand as trials progress towards larger volumes, avoiding the need for costly reinvestment.
Improved Quality Assurance – automation reduces human error, ensures consistent dosing or filling, and provides the digital records required for regulatory approval.
Faster Timelines – rapid setup and changeover features directly support accelerated trial schedules, helping organisations remain competitive in bringing new therapies to market.
The Role of SP Automation & Robotics
For over 40 years, SP Automation & Robotics has supported the pharmaceutical industry with advanced automation solutions. We understand that clinical trial environments demand far more than scaled-down production machinery. They require intelligent, agile and compact systems that deliver performance without compromise.
Our approach involves:
Collaborative Design – we work closely with pharmaceutical teams to understand specific trial requirements, from facility layouts to regulatory considerations.
Prototyping and Validation – before full deployment, systems are rigorously tested and validated to ensure compliance and reliability.
Integration with Data Systems – our equipment supports full digital traceability, ensuring every unit produced meets audit and compliance standards.
Lifecycle Support – from installation to ongoing maintenance and adaptation, we partner with our clients throughout the entire lifecycle of the equipment.
Applications of Clinical Trial Equipment
Bespoke automation plays a role across a wide range of trial-related processes, including:
-
Filling and packaging systems for small batches.
-
Serialisation solutions to ensure traceability and patient safety.
-
Automated dispensing systems for precise dosing.
-
Rapid changeover designs to enable flexibility across multiple protocols.
-
Compact cleanroom compatible systems that meet sterile and aseptic requirements.
By delivering proven reliability across these processes, SP Automation & Robotics helps trial teams focus on science and patient outcomes, rather than equipment limitations.
The Strategic Value of Investing in Bespoke Equipment
Clinical trials are not only scientific endeavours but also strategic investments, and the choice of equipment has far-reaching consequences. The right systems can accelerate regulatory approval by embedding data integrity and compliance into every process from the outset. They also play a vital role in reducing risk by minimising variability in production, ensuring that results are both reliable and repeatable.
Beyond efficiency, bespoke equipment contributes to reputation. Delivering consistent quality reinforces trust with regulators, reassures investors, and strengthens relationships with stakeholders across the healthcare sector. Equally important is the ability to support future growth. Equipment designed with scalability in mind provides a clear pathway from trial-scale manufacturing to full commercial production, smoothing what is often a challenging transition.
Ultimately, the cost of ineffective equipment cannot be measured only in pounds. It is also measured in the treatments that are delayed and the opportunities for patients that are lost when systems are not fit for purpose.
Moving away from A-B-C manufacturing
As technology continues to evolve, SP Automation & Robotics develops innovative solutions to keep pace. In traditional manufacturing, products move along a fixed sequence of steps, for example, from Station A (first operation) to Station B (next operation), then Station C, and so on. This rigid “A → B → C” flow works when every product is identical and runs are long.
But today, manufacturers face increasing demand for customisation and smaller batch sizes, which makes fixed paths less efficient. To solve this, SP Automation & Robotics leverages advanced transport technologies like Beckhoff’s XPlanar and XTS. These systems allow products to move intelligently and flexibly between stations, skipping or reordering steps as needed.
Instead of always going through every stage in order (A → B → C), a product might move directly from A to Z to F, or follow a different route like C → F → G. In other words, the “letters” simply represent different process stations, and these new technologies let manufacturers unlock countless possible paths through them, making production far more adaptable.
Looking Ahead: The Future of Clinical Trial Manufacturing
The pharmaceutical sector is evolving rapidly. Personalised medicine, advanced biologics and cell and gene therapies are reshaping how trials are conducted. These innovations require equipment that is more flexible, precise, and integrated than ever before.
Automation will be central to meeting these challenges. At SP Automation & Robotics, we envision the future as one where bespoke, intelligent systems support not only production but also data analytics, digital compliance, and real-time monitoring. By investing in these capabilities today, pharmaceutical companies can prepare for the complexity of tomorrow’s trials.
Conclusion
Clinical trials are the proving ground of pharmaceutical innovation. They demand agility, precision, compliance and efficiency in equal measure. Bespoke equipment, designed for the specific requirements of trial environments, is essential for overcoming the challenges that standardised systems cannot address.
SP Automation & Robotics is proud to be a trusted partner to the pharmaceutical industry. Our clinical trial equipment combines decades of engineering expertise with a forward-looking approach to automation, helping organisations deliver therapies to patients faster, more reliably and with complete confidence.
When the future of medicine depends on the outcome of clinical trials, the role of equipment cannot be underestimated. With the right solutions in place, progress becomes not only possible but predictable.
Follow us on LinkedIn to stay updated. Read more about our automation here…
- Benefits of Automation
- Key Considerations for Integrating Vision Systems into Pharmaceutical Machinery
- Revolutionising the Pharmaceutical Industry with Automation and Robotics
- Automated Medical Device & Assembly Systems
- Vision Systems for Pharmaceutical Machines: Types and Applications
- Manufacturing Problems Pharmaceutical Automation Can Help Solve
- Cost of Industrial Automation
- Advantages & Disadvantages of Robotic Automation
- Medical Device Automation
- Bowl Feeder vs. a Vision Guider Feed System
- Special Purpose Machinery
Contact Us
More information
Here you can find the most important news, blogs and videos from SP Automation & Robotics.. We will highlight developments, insightful industry trends, company announcements, technology expos, conferences, and events.